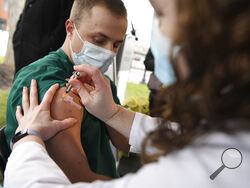
WASHINGTON (AP) — Hundreds more U.S. hospitals will begin vaccinating their workers Tuesday as federal health officials review a second COVID-19 shot needed to boost the nation’s largest vaccination campaign.
Packed in dry ice to stay at ultra-frozen temperatures, shipments of Pfizer’s COVID-19 vaccine are set to arrive at 400 additional hospitals and other distribution sites, one day after the nation’s death toll surpassed a staggering 300,000. The first 3 million shots are being strictly rationed to front-line health workers and elder-care patients, with hundreds of millions more shots needed over the coming months to protect most Americans.
The Food and Drug Administration is set to publish its analysis of a second rigorously studied COVID-19 vaccine, which could soon join Pfizer-BioNTech’s in the fight against the pandemic. If FDA advisers give it a positive recommendation on Thursday, the agency could greenlight the vaccine from drugmaker Moderna later this week.
A second vaccine can’t come soon enough as the country’s daily death count continues to top 2,400 amid over 210,000 new daily cases, based on weekly averages of data compiled by Johns Hopkins University. The devastating toll is only expected to grow in coming weeks, fueled by holiday travel, family gatherings and lax adherence to basic public health measures.
The first vaccine deliveries have provided a measure of encouragement to exhausted doctors, nurses and hospital staffers around the country.
Johnnie Peoples, a 43-year-old survival flight nurse, was excited and a little nervous Monday afternoon as he unzipped his flight suit and stuck out his left arm to become the first person to receive the vaccine at the University of Michigan Medical Center in Ann Arbor.
“Just to be a part of it is a good feeling,’’ he said.
Since March, he’s transported critically ill COVID-19 patients by jet from smaller hospitals around the state to the university medical center. It’s up-close-and-personal work that requires him to adjust ventilator settings and administer infusions to keep blood pressure from plummeting.
In Florida, government officials expect to have 100,000 doses of the vaccine by Tuesday at five hospitals across the state.
“This is 20,000 doses of hope,” said John Couris, president and chief executive officer, Tampa General Hospital, after the delivery of 3,900 vaccine vials on Monday. Each vial has five doses.
Because the vaccine requires two rounds, the people getting injections now will need a second shot in about three weeks.
Vaccinations were also expected to kick off Tuesday in New Jersey, which is dividing some 76,000 doses among health workers and nursing home residents. The federal government is coordinating the massive delivery operation by private shipping and distribution companies based on locations chosen by state governors.
Following another initial set of deliveries Wednesday, officials with the Trump administration's Operation Warp Speed in Washington said they will begin moving 580 more shipments through the weekend.
“We’re starting our drumbeat of continuous execution of vaccine as it is available,” Army Gen. Gustave Perna, chief operating officer for Warp Speed, told reporters Monday. “We package and we deliver. It is a constant flow of available vaccine.”
Shots for nursing home residents won’t begin in most states until next Monday, when some 1,100 facilities are set to begin vaccinations.
Perna and other U.S. officials reiterated their projection that 20 million Americans will be able to get their first shots by the end of December, and 30 million more in January.
That projection assumes swift authorization of the vaccine up for review this week, co-developed by Moderna and the National Institutes of Health. Like the Pfizer-BioNTech vaccine, Moderna's requires two shots for full protection.
Last month, Moderna and NIH reported that their shot appeared to be nearly 95% effective across various ages and racial groups, according to results from an ongoing 30,000-person study. The main side effects were fatigue, muscle aches and injection-site pain after the second dose. Those flu-like reactions are common to many vaccines and are a sign the vaccine is revving up the immune system to help fight off the virus.
Moderna reported no major safety problems from its study. But FDA’s panel is certain to scrutinize the data for any indications of possible severe allergic reactions or other rare side effects. Officials in the U.K. are investigating several adverse reactions there with Pfizer’s vaccine and FDA is closely monitoring the rollout here for similar reports.
Both Moderna’s and Pfizer-BioNTech's shots are so-called mRNA vaccines, a brand-new technology. They aren’t made with the coronavirus itself, meaning there’s no chance anyone could catch it from the shots. Instead, the vaccine contains a piece of genetic code that trains the immune system to recognize the spiked protein on the surface of the virus.
___
Associated Press writers Lindsey Tanner, Tamara Lush, Candice Choi and Lauran Neergaard contributed to this report.